Understanding Clinical Trials
Clinical trials are carefully designed research studies that help doctors and scientists understand whether new medical treatments, medications, or devices are safe and effective for people.
They are a critical part of advancing medicine — every vaccine, medication, or therapy available today was once tested through a clinical trial.
The Purpose of Clinical Trials
Clinical trials aim to answer important questions such as:
- Does a new treatment work better than current options?
- What are the potential side effects or risks?
- How does the treatment affect different groups of people?
- What is the best way to deliver or dose a medication?
By participating in a clinical trial, patients help shape the future of healthcare and make it possible for others to benefit from better treatments down the road.
Phases of a Clinical Trial
Clinical trials happen in stages, called phases, each with a specific goal:
- Phase I – Tests safety and dosage in a small group of volunteers.
- Phase II – Focuses on how well the treatment works and gathers more safety data.
- Phase III – Compares the new treatment to the current standard of care in larger groups.
- Phase IV – Conducted after approval to continue monitoring long-term safety and effectiveness.
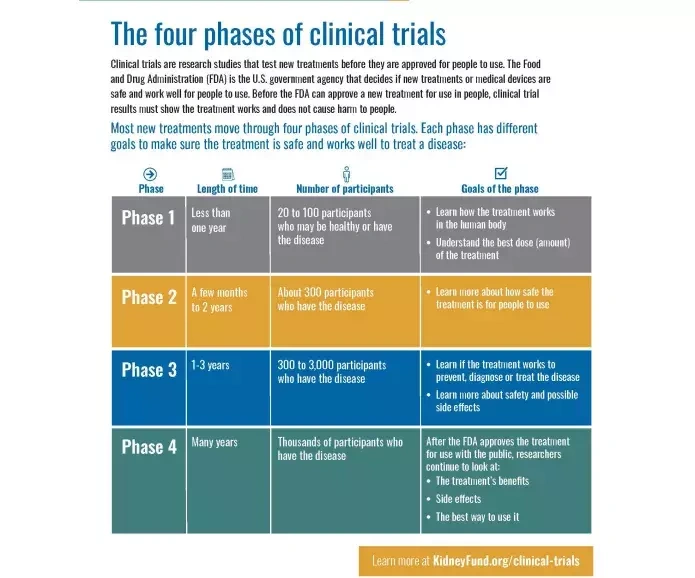
Image Source: American Kidney Fund
Why Clinical Trials Matter
Clinical trials drive medical progress. They help:
- Discover better ways to prevent, diagnose, and treat diseases
- Improve patient outcomes and quality of life
- Ensure that new treatments are safe and effective for real-world use
Without volunteers, clinical research wouldn’t be possible. Every participant plays a vital role in moving medicine forward — not just for themselves, but for future generations.
How You Can Get Involved
If you’re interested in participating in a clinical study, talk to your healthcare provider or reach out to our clinical research team. We’ll help you understand:
- What studies are currently enrolling
- What participation involves
- How your safety and privacy are protected
Clinical trials are always voluntary, and participants can withdraw at any time.

Clinical trials provide essential pathways for medical advancement that benefit communities worldwide. Our guide has explored the structured approach these research studies follow to ensure participant safety while advancing healthcare options.
Your participation in clinical research holds tremendous value for medical progress. Each volunteer contributes to discoveries that shape treatment standards and improve patient outcomes. We emphasize that participant rights and safety remain our primary concerns, supported by ethical guidelines, informed consent processes, and withdrawal freedoms.
Verity Health offers extensive support for patients considering clinical research opportunities. Our team brings over 100 years of combined clinical experience to guide you through research participation decisions. We look forward to providing state of the art clinical therapy and research guidance to the community of Vancouver WA and surrounding areas.
Our vision extends to empower the community’s wellbeing through multiple health services. We provide innovative treatments including FibroScan for liver assessment, GLP-1 medications for weight management, and Ultraslim for body contouring. These services complement our clinical research offerings, fostering balanced lifestyles and envisioning a healthier future.
Whether you choose clinical research participation or explore our other health services, taking active roles in your healthcare journey empowers better outcomes. We stand ready to support your path toward optimal wellbeing, offering the guidance and expertise needed for informed health decisions.
Ready to Take Control of Your Liver Health?
If you’re concerned about your liver, fatty liver disease, or metabolic health, our board-certified gastroenterologists are here to help.
Schedule an appointment to get a clear diagnosis and a personalized plan for a healthier life.
FAQs
Q1. What are the main phases of clinical trials? Clinical trials typically progress through four main phases. Phase 1 tests safety in a small group, Phase 2 evaluates effectiveness and side effects, Phase 3 compares the new treatment to standard options, and Phase 4 monitors long-term effects after approval.
Q2. How long does each phase of a clinical trial usually last? The duration varies by phase. Phase 1 usually lasts several months, Phase 2 can take several months to 2 years, Phase 3 typically runs for 1 to 4 years, and Phase 4 involves ongoing monitoring after the treatment is approved.
Q3. What rights do participants have in clinical trials? Participants have the right to informed consent, which means receiving clear information about the trial’s purpose, procedures, risks, and benefits. They also have the right to withdraw from the trial at any time without penalty or loss of standard care.
Q4. How are participants’ safety ensured during clinical trials? Safety is ensured through multiple measures, including thorough laboratory testing, ethics committee approvals, and careful monitoring throughout the trial. Independent review boards oversee all trials, and Data and Safety Monitoring Boards provide additional oversight.
Q5. Why are clinical trials important for medical progress? Clinical trials are crucial for developing new treatments, drugs, and medical devices. They help researchers determine if new approaches are safe and effective before making them widely available to patients. Without clinical trials, many life-saving medical advancements would not be possible.